Abstract
Background
The Highlands of Papua New Guinea are non-endemic for malaria compared to the rest of the country. This study aimed to explore the local transmission of malaria in the Highlands through a cross-sectional school survey coupled with reactive case detection.
Methods
Between July and November 2019, 5575 schoolchildren and 1048 household members were screened for malaria using Rapid Diagnostic Tests, subsequently validated by light microscopy. In addition, an analysis of malaria cases (2017 to 2019) was conducted across 33 health facilities within the catchment areas of the surveyed schools and households.
Results
Thirteen individuals were diagnosed with malaria: eleven with Plasmodium falciparum (five schoolchildren, six household members) and two with Plasmodium vivax (one student, one household member); all were aged ten years or older. Malaria prevalence was 0.09% [95% CI 0.03, 0.3] among schoolchildren and 1.7% [95% CI 0.3, 9.1] among household members. Eleven positive individuals (84%) reported recent travel, mainly to lower-altitude endemic areas. Long-Lasting Insecticidal Nets were used by 34.8% [95% CI 28.7, 40.8] of household members. The average annual malaria incidence in the catchment areas was 3.7 cases per 1000 [95% CI 2.6, 5.3] among the general population, while children under 15 years accounted for 19% [95% CI 14, 27] of the positive cases.
Conclusions
Local malaria transmission appears to be minimal in the surveyed Highlands areas. Strengthening surveillance-response system to control imported cases and stop local foci could support malaria elimination in PNG. However, effective operational triggers for reactive case finding remain to be determined.
Similar content being viewed by others
Background
Malaria is endemic throughout Papua New Guinea (PNG) except in the Highlands, where occasional epidemics occur in certain areas [1,2,3]. While four species of Plasmodium are known to circulate in PNG, Plasmodium falciparum causes most malaria infections in the country, followed by Plasmodium vivax [4, 5]. With the heterogeneous geography of PNG, varieties of mosquitoes mostly belonging to the Anopheles punctulatus group have adapted to different ecological niches and maintain malaria transmission [6].
Located centrally in the mainland of PNG is the Highlands Region, which covers about 463,000 km2, or 16% of PNG’s total land area. This region is characterized by the Central Range (reaching above 4,500 m) with deep valleys where agricultural activities occur, and neighbouring endemic coastal areas. The Region is divided into seven provinces: Southern Highlands, Hela, Enga, Western Highlands, Jiwaka, Chimbu, and Eastern Highlands. According to the 2011 National Census, the Highlands Region had a population of 2.85 million, making up 39% of the entire population of PNG [7].
Previous studies on malaria in the Highlands showed a negative correlation between malaria prevalence and altitude, especially during non-epidemic periods [1,2,3]. Areas above 2000 m are considered non-receptive for malaria transmission, regions at altitudes 1200–2000 m are considered as epidemic-prone, and areas below 1200 m as endemic [3]. A recent stratification of the incidence of malaria (2011–2019) in PNG revealed that over 95% of the population in the Highlands live in areas of very low-to-low risk [3].
The National Malaria Control Programme (NMCP) has been relying on various interventions including distributions of long-lasting insecticidal nets (LLINs), prompt diagnosis and treatment with artemisinin-based combinationtherapy (ACT), and home management of malaria (HMM) in selected areas. After a significant reduction of malaria burden, achieved with Global Fund support that started in 2004, a resurgence has been reported in PNG since 2014 [8, 9]. However, the increase is not uniform, and malaria risk has remained low in the Highlands. In 2016/17, the Malaria Indicator Survey (MIS) had reported infections in only three villages (out of 20) in the region. Notably, none of the eight malaria cases (out of 1442 tested persons) was in children under five years, suggesting importation of infections rather than local transmission [8, 10]. In 2015, partly due to funding shortfalls, the NMCP and Rotarians Against Malaria (RAM) stopped the distribution of LLINs in areas above 2000 m and targeted only children under five years above 1600 m [3].
As PNG aims to progress towards malaria elimination, a confirmation of the very low malaria prevalence in the Highlands was required to support sub-national tailoring of interventions, such as the change in the LLIN strategy, in light of very limited resources.
School malaria surveys (SMS) target children who are likely to be stationary than adults, who may acquire an infection elsewhere. SMS has gained increased attention for national surveillance, complementing household surveys including MIS. Schools are often well organised, easily accessible, and provide the opportunity of collecting malariometric data from many children in a short period of time and at a low cost compared to Demographic and Health Surveys (DHS) or MIS [11, 12]. As a result, SMS can be useful for understanding local transmission and planning targeted interventions [11].
In order to investigate local malaria transmission in the Highlands and generate evidence to optimise the implementation of preventive and curative interventions, an SMS was conducted in the region. Prevalence of malaria in schoolchildren aged 5–16 years and associated travel history information were used to establish a proxy measure for locally acquired malaria infections. In addition, a Reactive Case Detection (RCD) survey at the household level aimed to confirm whether positive schoolchildren were indicative of local transmission clustered in their households. Previous studies showed that malaria cases cluster in space at the household level, near breeding sites, and are affected by human and mosquito behaviours [13, 14]. Hence, RCD may be useful to identify clusters of malaria infections and investigate sources of transmission. The RCD approach has been used in several studies to find and treat asymptomatic malaria cases living near clinical index cases [15].
Methods
Selection of schools
A comprehensive list of operational primary (n = 784) and elementary (n = 1719) schools across the seven provinces was acquired from the National Department of Education, serving as a sampling frame for the selection of thirty schools along with two backups. Thirty schools were selected due to logistical and cost constraints. This number ensures the schoolchildren sample mean follows a normal distribution, based on the Central Limit Theorem (CLT). The School Malaria Survey (SMS) aimed to assess malaria prevalence among children aged 5–16 years; thus, pairs of elementary (grades 1 and 2) and primary schools (grades 3–8) were chosen to encompass this age range. The corresponding elementary school within the primary school's catchment area was selected. To account for the altitude effect on malaria prevalence, a stratified sampling approach was employed. Catchment areas of the schools were mapped using ArcGIS 10.3, and these delineated areas were grouped by malaria incidence categories. Hence, three incidence categories were defined: low (< 1), moderate (1–10), and high (> 10 per 1000). A weighted random sampling, utilizing a random number table in STATA, was then performed within each incidence category to select the schools.
Selection of schoolchildren, and households for RCD
Within each pair of schools, 200 schoolchildren were randomly sampled from a list of all students. Capping the total at the 200 was due to practical reasons given the time and resources limits of the survey. In case a sampling frame contained less than 200 individuals, all available students were selected. Then, sampled schoolchildren were tested for malaria as described below. Those who tested positive using RDTs were chosen for a subsequent RCD household survey. Additional households were randomly selected from the group of schoolchildren who tested negative, bringing the total to ten households per school. Every present household member was then tested for malaria.
Data collection forms
In the SMS, three data collection forms were utilized: a school summary form, a malaria test register, and a student questionnaire. The first two forms were filled out once per school by the team leader and research nurse, while the third form, an electronic structured questionnaire was administered to the selected schoolchildren. Additionally, during the RCD survey, three electronic questionnaires were employed, aimed at the household head and other members. These questionnaires, adapted from the Malaria Indicator Survey set [16], included a household questionnaire (one per household), a treatment-seeking questionnaire (one per household member with a recent febrile illness), and a prevalence form (one per household member tested for malaria), all programmed in Open Data Kit (ODK) at PNGIMR and administered via tablet computers.
Survey implementation procedures
The SMS and RCD were conducted from July to November 2019 by three PNGIMR field teams operating concurrently at different locations. Each team comprised two nursing officers, a scientific officer, and one or two research assistants, all of whom underwent comprehensive training on the project background, survey protocol, and sampling techniques.
Upon arriving at a selected school, an informational meeting with parents and the school board was arranged to explain the study and its methods. RCD interviews were held with household heads, while all household members provided blood samples and those with a recent fever episode were interviewed on treatment seeking. The survey spanned 6–7 days per school (including RCD households). Handheld GPS devices (Garmin) were used to log the geo-coordinates and elevations of the surveyed schools and households.
Testing of malaria and anaemia
Trained nurses collected blood samples by finger-prick from study participants (i.e., selected schoolchildren and household members) aged six months or older. One thick and one thin smear were prepared on the same glass slide for malaria diagnosis by light microscopy. In addition, a rapid diagnostic test (RDT)(CareStart Malaria HRP2/pLDH (Pf/pan) Combo Test, Access Bio) was performed, and a microcuvette sample was prepared to measure haemoglobin (Hb) levels using a handheld HemoCue Hb 201 + analyser (HemoCue, Angelholm, Sweden).
During the surveys, positive RDT results were classified as P. falciparum (control line with HRP2 test-line only), non-P. falciparum (control line with pLDH test-line only), or P. falciparum or mixed infection (control line with two test lines for HRP2 and pLDH) [17]. If an invalid RDT result was encountered (i.e., no control line, irrespective of the two test lines), the test was repeated.
The axillary temperature of participants was measured with an electronic thermometer, an acute fever was defined as exceeding 37.5 °C. In addition, children aged (2–9) years were examined for splenomegaly and graded according to the Hackett grading system [18]. The nursing officers provided treatment for RDT positive individuals following the national treatment protocol. They also offered treatment for other minor ailments encountered or gave referral advice.
Light microscopy was done at the PNGIMR in Madang following established procedures for research studies [5, 19]. Two qualified microscopists had examined blood slides independently and were blinded to results of each other. Slides were considered positive for malaria if judged positive by at least two microscopists. A third senior microscopist examined slides with discrepant results.
Passive case detection in the nearby health facilities
Data on malaria cases reported in the health facilities (HFs) between 2017 and 2019 was sourced from the electronic Health Information System (eNHIS). Since the obtained dataset does not provide information on malaria cases at the aid post level, the analysis was limited to the nearest health centres and urban clinics. Nearest HFs to the surveyed schools and households were identified using shapefiles of the surveyed sites and health facilities in the Highlands, and a travel friction raster, as described in [3]. The gdistance package in R was employed to calculate cost distance of travel time (minutes) from surveyed sites to HFs.
Only cases confirmed by RDTs or microscopy at the HFs were included in the analyses. Malaria incidence within the catchment areas of surveyed schools and households was computed for the general population, as well as for the children under five years and children at school age (5–14 years). The population projections for the three years, based on 2011 Census and growth rates reported by the National Statistics Office of PNG were utilized in incidence estimations. Given that the annual incidence rates exhibit over-dispersion, negative binomial models were employed to estimate the average incidence rates, along with 95% confidence intervals.
Data management and analysis
Data were collected electronically using the ODK Collect application installed on tablet computers. Completed and verified collected forms were uploaded directly to the project server at the Swiss TPH using the local mobile phone network (Digicel). ODK Briefcase v1.4.9 was used to download the data and export it for analysis in STATA/IC 14.2 (StataCorp LLC, College Station, TX, USA).
Weighted statistics with 95% confidence limits were calculated using the survey design command set in Stata (svy). Schools and households were established as the primary sampling units, while the seven provinces were treated as strata. Separate sampling weights were calculated for schools and household surveys based on the inverse of an observation's probability of selection. The overall selection probability was determined from the eight combination categories of altitude and incidence within the primary schools' sampling frame. For each school, the selection probability of a RCD household was computed among tested schoolchildren. Since all members of the sampled family were eligible, individual-level weights were set equal to the household weights to which an individual belonged.
Malaria prevalence was calculated separately for tested schoolchildren and household members as a proportion of positive results detected by light microscopy. WHO adjustments of Hb measurements, considering sex and altitude [20] were applied prior to determining the anaemia status of each tested individual and calculation of prevalence among the study groups. Prevalence of malaria and anaemia were age-standardised using the overall age distribution of the PNG population reported from the 2011 Census by the National Statistics Office (NSO) of PNG.
Contingency tables and odds ratios with 95% confidence intervals were generated separately for the schoolchildren and household groups to explore the relative risk of malaria infection by travel history, ownership, and use of bed nets. Where appropriate, Microsoft Excel graphs of percentage and cumulative frequencies were used to compare the participants' groups. Further, to account for the relationship of altitude and malaria transmission, four altitudinal categories were used in the analyses in line with previous publications [3]: < 1200 m, 1200–1600 m, 1600–2000, and above 2000 m.
LLIN ownership and use
Bed net ownership and use were calculated following standard procedures [21]. The population percentage with access to an LLIN was evaluated at the household level by creating an intermediate variable named the potential users of LLINs [22]. Hence, the number of LLIN sleeping spaces, assuming two per LLIN, was calculated as potential users, which was then divided by the number of people sleeping in the household the previous night. However, the access proportion was adjusted if the potential use exceeded the number of people in the house the last night, i.e., to converge the access proportion to one. Net use among the people with access to LLINs was calculated by dividing the number of people using an LLIN by the total population with access (multiplying the weighted proportion with access by the total population).
Results
Surveyed schools
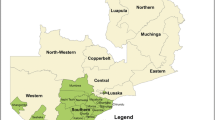
Thirty pairs of primary schools along with their feeder elementary schools (30 + 30) were selected across the Highlands provinces (Fig. 1). The altitudes of the surveyed schools ranged from 937 to 2409 m above sea level. Within a 5 km radius surrounding these schools, the minimum altitudes were less than 1600 m in the catchment areas of 18 schools, but none of the surveyed schools or adjacent villages were located at altitudes below 800 m.
Locations of surveyed schools in the Highlands, PNG 2019. The thirty primary schools: 1 Yauna, 2 Shinler, 3 Gotumi, 4 Kafetina, 5 Gorohanota, 6 Silma, 7 Goglme, 8 Gon, 9 Keram, 10 Anglimp, 11 Ageka, 12 Rebiamul, 13 Minimp, 14 Kotna, 15 Arawagai, 16 Dalapana, 17 Yano, 18 Minamb, 19 Par, 20 Sakarip, 21 Pumakos, 22 Yalis, 23 Margarima, 24 Ugu, 25 Lake Kutubu, 26 Tubo, 27 Poroma, 28 Fogomau, 29 Apenda, 30 Tunda
SMS and RCD survey participants
Table 1 presents the total numbers of individuals sampled in the SMS and RCD surveys. The SMS encompassed 5775 schoolchildren hailing from 237 different villages. Additional information on surveyed schoolchildren by province and age is provided in Supplementary Table S1.
The 1,048 participants in the RCD belonged to 296 households in 106 villages (i.e. a subsample of the 237 villages). Approximately half of the household heads engaged in subsistence activities, including farming and fishing. Other characteristics of RCD households, such as their main sources of lighting, drinking water, and income, detailed in Supplementary Table S2.
Malaria prevalence
Overall, malaria prevalence by light microscopy was very low among the schoolchildren (0.09%; 95% CI 0.03, 0.3) and household members (1.7%; 95% CI 0.3, 9.1). Plasmodium falciparum infections were predominant, 11 of 13 positive cases, while only two infections of P. vivax were detected, Table 2. The prevalence of P. falciparum in the SMS and RCD surveys was therefore 0.1% [95% CI 0.0, 0.3] and 1.5% [95% CI 0.2, 9.8], respectively. These infections were identified in four provinces: Hela (n = 6), Enga (n = 5), Eastern Highlands (n = 1) and Western Highlands (n = 1). The age of all positive cases was above ten years.
Further, microscopy and RDTs showed 100% concordance, i.e. the 13 infections were detected by both methods. The microscopy analysis identified the composition of positive strips of RDTs as nine P. falciparum, three non-falciparum, and one strip of P. falciparum or mixed infection.
For additional details of positive malaria cases detected in the SMS and RCD surveys, see Supplementary Table S3.
None of the positive schoolchildren had a positive individual in their household. The four households with positive members belonged to negative schoolchildren. Similarly, there was mismatch at the village scale, i.e., infected schoolchildren did not originate from villages with positive households. For distribution of household members and locations with positive cases of schoolchildren and non-students, see Supplementary Figure S1.
Among the 296 households visited for RCD, participants with a malaria infection were located in only four out of 137 villages, all above 1600 m, with a single exception of a residence at 1529 m in the Eastern Highlands Province. Conversely, 46.2% (n = 132) of the negative households were situated in 59 villages at altitudes below 1600 m.
Fever, anaemia and splenomegaly
All thirteen individuals who tested positive for malaria in this study, except one, were found to be asymptomatic, exhibiting no fever in the last two days or other common signs (such as chills, headache, vomiting).
All individuals tested positive for malaria by microscopy were non-anaemic with normal spleens examinations. The prevalence of anaemia among the schoolchildren was 14.8% [95% CI 10.3, 19.2]. In contrast, a higher prevalence of 23.3% [95% CI 20.2, 26.9] was observed among RCD household members. There was also a notable variation in anaemia prevalence across different provinces, particularly between Jiwaka (where it was most common) and Chimbu (where it was least common), as depicted in Supplementary Figure S2. Severe anaemia, defined as a haemoglobin level of less than 8 g/dl, was found in only 0.1% of the schoolchildren and 0.3% of the household members. Among the 1131 schoolchildren aged 5–9 years who were examined for splenomegaly, only 2.2% [95% CI 0.4, 11.7] exhibited signs of splenomegaly, as defined by a Hackett score of 1–5. Moreover, none of the 227 household members aged 2–9 years who had their spleen palpated had an enlarged spleen.
Travel history and risk of malaria
There is no travel history reported by 83% (N = 4790) of the schoolchildren and 90% (N = 934) of the household members. Among the participants who travelled, endemic coastal provinces are the destination for 23% (N = 231) of the schoolchildren and 40% (N = 39) of the household members. Intra-provincial travel or travel to other Highlands provinces accounted for 67% (N = 759) and 60% (N = 75) of the travel undertaken by schoolchildren and household members, respectively.
Notably, 11 of the 13 cases (84.6%) reported travel within the previous month. Specifically, a cluster of four positive individuals living in Margarima station (Hela) disclosed prior visits to three low-altitude locations. Interestingly, two individuals with P. falciparum infections resided at elevations above 2000 m in Enga Province, but did not report a recent travel history. In contrast, only 17.1% schoolchildren and 10.8% household members reported travel in the previous month among who tested negative; see Supplementary Table S4.
Ownership and use of bed nets
Table 3 shows the ownership and use of bed nets by the altitude of RCD households. There is a decrease in household ownership and use of LLIN as altitude increases. Moreover, a substantial disparity in bed nets accessibility by individuals is observed in households above 1200 m compared to those below this elevation, 47% and 87%, respectively. Nevertheless, among the thirteen positive cases, 85% reported that they do not have a bed net in the house, and none slept under a net the previous night (Supplementary Table S5 and Table S6).
Treatment-seeking behaviour by sick individuals
Overall, 11.6% (668/5775) of the interviewed students reported school absenteeism due to illness within the preceding two weeks. Within this subset, 90% (603/668) reported experiencing a fever, yet 62% (372/603) sought medical attention at HF, with 8% (50/603) being diagnosed with malaria. In contrast, 3.6% (38/1038) of the household members had reported recent fever symptoms, but only 34% (13/38) visited a HF for treatment.
Among the six positive cases of the schoolchildren, three reported absenteeism from the school during the last two weeks due to illness. Two of the sick schoolchildren sought medical care at a HF but neither was diagnosed with nor treated for malaria. Likewise, in the RCD survey, the positive household members did not report any illness nor did they seek treatment in a HF.
Malaria incidence in nearby health facilities (HFs)
Overall, 33 HFs were identified in the catchment areas of the surveyed schools and households (Supplementary Table S7). Three HFs accounted for 66% (N = 11,091) of reported malaria cases: Tinsley Health Centre (38%), Rebiamul Urban Clinic (19.8%) and Kundiawa Hospital (8.2%). Three HFs reported no malaria infection among children 5–14 years, while ten HFs documented no cases in children under 5 years. Among the confirmed cases, 8797 (79%) were infections of P. falciparum as diagnosed by microscopy or RDTs, including (potentially) mixed infections.
The average annual malaria incidence (2017 and 2019) was 3.7 cases per 1,000 individuals [95%CI 2.6, 5.3] among the general population. Figure 2 shows incidence and positivity rates of malaria plotted by altitude and proportion of positive children. Higher incidences and positivity rates mainly corresponded to the large number of cases in Tinsley and in areas below 2000 m. The average percentage of positive cases among children relative to all positive cases was 19.1% [95% CI 14.1, 26.7], but higher proportions are noticed in areas between 1400 and 1800 m.
Discussion
The SMS investigated a proxy measure for local transmission in the Highlands, assuming schoolchildren aged 5–16 years are less likely to travel away from their home than older age groups. The few positive cases of infection detected in the schoolchildren and members of RCD households correspond well with the low incidence rates in 2017–2019, suggesting minimal local malaria transmission in surveyed areas. According to WHO criteria for stratification of transmission intensity [23], a very low transmission is characterized by an annual parasite incidence of < 100 cases per 1000 population and prevalence of P. falciparum/P. vivax > 0 but < 1%. Hence, the prevalence and incidence rates established in this study indicate very low transmission, suggesting conducive conditions to accelerate towards local elimination in the Highlands of PNG.
In the subsequent Malaria Indicators Survey 2019/2020, malaria infections were found in only two villages (out of 40) in the Highlands, with a prevalence 0.03% using light microscopy and 0.3% when considering RDTs [10]. In a survey early 2000s, Mueller and others reported prevalence of > 10% during epidemic outbreaks in 11 villages at 1400–1700 m in the Highlands [2]. In comparison, the results show lower prevalence proportions (< 1%) in all surveyed schools and villages. However, the SMS was conducted during the dry season (July and November), i.e., an unlikely suitable time for epidemics.
The two infections in schoolchildren without travel history in Enga (two places: Minamb and Par) remain difficult to explain. After completing the survey, the heads of households were contacted to recheck any travel during the past year by their children. However, they asserted there had been no trip during that period. One of the design limitations of this work is that respondents do not report day trips as travel history, especially if there was no overnight stay in the visited places. Mapping day-movements between high and low-risk areas might provide additional insights about the risk of malaria importation associated with day trips.
This study demonstrated the feasibility of implementing RCD as a reactive focal search for malaria infections in certain areas of the Highlands of PNG. However, following up schoolchildren with a malaria infection at their home did not result in the detection of additional infections. This finding may indicate a lack of clustering of malaria cases at households of infected schoolchildren, for example because transmission did not occur near their homes, supporting the conclusion of limited local transmission. Similarly, cases among schoolchildren may not be suitable index cases to direct re-active searches for clusters of malaria infections, particularly as most infected schoolchildren had a travel history. In the low-transmission settings of Zanzibar [14] and Namibia [24], symptomatic malaria patients detected passively at health facilities were useful to guide reactive focal interventions and detect clustered infections in their households. It remains to be determined whether clinical index cases are a more suitable operationally trigger for re-active case finding in the Highlands of PNG.
Malaria microscopy was used to confirm the results of RDTs and provide accurate species-specific diagnoses. Individual discrepancies, e.g. the case identified as non-P. falciparum by RDTs and as P. falciparum by light microscopy, may be related to the sensitivity of RDTs in detecting low-density infections [17, 25]. The detection limit of RDTs (and conventional light microscopy) of approximately 100 parasites/μl blood may have resulted in an under-estimation of the malaria prevalence in this study. However, while this has been a major limitation to the effectiveness of RCD in previously high endemic settings [25], in the Highlands of PNG immunity against malaria is expected to be largely absent (except maybe in frequent travellers to the coast), making it less likely that infections in the local population remain low-density, asymptomatic, and hence un-detected. In contrast, detection of low-density infections could require the use of molecular surveillance tools to eliminate residual cases.
One of the main limitations of this study is under-sampling in some parts of the Highlands, mainly Jimi Valley (Jiwaka and Western Highlands provinces), Karimui (Chimbu), Obura-Wonenara (Eastern Highlands), Lake Kopiago (Southern Highlands), Mt. Bosavi Rural (Hela), and Lagaip/Pogera (Enga). In the latter two areas, ongoing tribal fighting forced the survey teams to withdraw and replace the selected schools using the backup list. Other areas such as Obura-Wonerra and Lake Kopiago were inaccessible due to flooding during the survey time. Conducting an SMS in these zones at a suitable time could provide valuable additional insights, especially as these areas might be receptive to malaria transmission based on evidence from previous surveys [1]. Also, these epidemiological surveys should be combined with entomological surveys – a limitation to this study – to better understand the role of local malaria vectors in the Highlands.
Accelerating efforts towards malaria elimination in the Highlands of PNG requires measures to prevent local transmission and control the importation of infections from endemic areas in the lowlands. A previous study already reported 82% of malaria cases in Goroka to be associated with travel to coastal areas [26]. While less than 20% of participants in this study reported travel during the previous month, most of these trips occurred within the Highlands Region. Endemic coastal provinces were the destination for only 10% to 17% of travelled survey participants in the SMS and RCD. As the Highlands become more connected with endemic provinces through infrastructural developments, the risk and potential severity of outbreaks associated with importation may increase in the non-immune population [1].
In a pre-elimination phase, it is therefore essential for the malaria control program to establish an effective surveillance-response mechanisms as an integral component of the suite of interventions, with an approach that allows tracing the source of reported infections followed by routine focal interventions, particularly where local transmission persists. With few cases in the Highlands, a decentralized malaria programme can implement both active and reactive investigation and clearance of foci to interrupt transmission. However, such a surveillance-response system requires individual case-based reporting including a thorough examination of detected cases to determine the source of infection. In addition, this system requires sufficient human resources as well as financial and logistical capacity to investigate transmission foci, including, for example RCD. Case-based surveillance will be an indispensable tool to prevent reintroduction in settings freed of malaria [27]. Establishing such a system may require specific structural changes in the national malaria policy to leverage needed resources from both national and sub-national (province, district) levels.
Conclusions
Findings from this study identify an opportunity to accelerate towards malaria elimination in the Highlands of PNG, from an epidemiological point of view. Following guidance provided in the WHO framework for malaria elimination, the PNG malaria programme should invest in sub-national tailoring to implement interventions along the existing continuum of malaria transmission from high to very low [23]. In support of targeted elimination efforts, further evidence on suitable triggers for reactive focal interventions are required alongside a continuous strengthening of the National Health Information System to include near-real-time individual case-based reporting of malaria cases.
Availability of data and materials
Data is provided within the manuscript or supplementary information files.
References
Betuela I, Maraga S, Hetzel MW, Tandrapah T, Sie A, Yala S, et al. Epidemiology of malaria in the Papua New Guinean highlands. Trop Med Int Health. 2012;17:1181–91.
Mueller I, Namuigi P, Kundi J, Ivivi R, Tandrapah T, Bjorge S, et al. Epidemic malaria in the highlands of Papua New Guinea. Am J Trop Med Hyg. 2005;72:554–60.
Seidahmed O, Jamea S, Kurumop S, Timbi D, Makita L, Ahmed M, et al. Stratification of malaria incidence in Papua New Guinea (2011–2019): contribution towards a sub-national control policy. PLoS Glob Public Health. 2022;2:e0000747.
Rodriguez-Rodriguez D, Maraga S, Lorry L, Robinson LJ, Siba PM, Mueller I, et al. Repeated mosquito net distributions, improved treatment, and trends in malaria cases in sentinel health facilities in Papua New Guinea. Malar J. 2019;18:364.
Hetzel MW, Morris H, Tarongka N, Barnadas C, Pulford J, Makita L, Siba PM, Mueller I. Prevalence of malaria across Papua New Guinea after initial roll-out of insecticide-treated mosquito nets. Trop Med Int Health. 2015;20:1745–55.
Cooper RD, Waterson DG, Frances SP, Beebe NW, Pluess B, Sweeney AW. Malaria vectors of Papua New Guinea. Int J Parasitol. 2009;39:1495–501.
Bourke RM, Allen B. Estimating the population of Papua New Guinea in 2020. Development policy centre discussion paper. SSRN J. 2021. https://doi.org/10.2139/ssrn.3770356.
Hetzel MW, Saweri O, Kuadima J, Smith I, Tandrapah A, Jamea-Maiasa S, et al. Papua New Guinea Malaria Indicator Survey 2016–2017: malaria prevention, infection, and treatment. Goroka, Papua New Guinea Institute of Medical Research. 2018:1–70.
Hetzel MW, Reimer LJ, Gideon G, Koimbu G, Barnadas C, Makita L, et al. Changes in malaria burden and transmission in sentinel sites after the roll-out of long-lasting insecticidal nets in Papua New Guinea. Parasit Vectors. 2016;9:340.
Seidahmed O, Jamea S, Tandrapah A, Timbi D, Hetzel M, Pomat W. Papua New Guinea Malaria Indicator Survey 2019–2020: final report on malaria prevention, infection prevalence, and treatment-seeking. Goroka, Papua New Guinea Institute of Medical Research. 2021:1–80.
Chacky F, Runge M, Rumisha SF, Machafuko P, Chaki P, Massaga JJ, et al. Nationwide school malaria parasitaemia survey in public primary schools, the United Republic of Tanzania. Malar J. 2018;17:452.
Gitonga CW, Karanja PN, Kihara J, Mwanje M, Juma E, Snow RW, et al. Implementing school malaria surveys in Kenya: towards a national surveillance system. Malar J. 2010;9:306.
Mogeni P, Williams TN, Omedo I, Kimani D, Ngoi JM, Mwacharo J, et al. Detecting malaria hotspots: a comparison of rapid diagnostic test, microscopy, and polymerase chain reaction. J Infect Dis. 2017;216:1091–8.
van der Horst T, Al-Mafazy AW, Fakih BS, Stuck L, Ali A, Yukich J, et al. Operational Coverage and Timeliness of Reactive Case Detection for Malaria Elimination in Zanzibar. Tanzania Am J Trop Med Hyg. 2020;102:298–306.
Aidoo EK, Afrane YA, Machani MG, Chebore W, Lawson BW, Atieli H, et al. Reactive case detection of Plasmodium falciparum in western Kenya highlands: effective in identifying additional cases, yet limited effect on transmission. Malar J. 2018;17:111.
Roll Back Malaria. Malaria Indicator Survey (MIS) Toolkit. 2014.
Maltha J, Gillet P, Bottieau E, Cnops L, van Esbroeck M, Jacobs J. Evaluation of a rapid diagnostic test (CareStart™ Malaria HRP-2/pLDH (Pf/pan) Combo Test) for the diagnosis of malaria in a reference setting. Malar J. 2010;9:171.
Hackett LW. Spleen measurement in malaria. J National Malar Soc. 1944;3:121–33.
TDR, WHO. Methods Manual: Microscopy for the detection identification and quantification of malaria parasites on stained thick and thin blood films in research settings. Geneva: World Health Organization; 2015.
WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Geneva: World Health Organization; 2011.
MEASURE Evaluation, MEASURE DHS, President’s Malaria Initiative, Roll Back Malaria Partnership, UNICEF, WHO. Household survey indicators for malaria control. 2013.
Kilian A, Koenker H, Paintain L. Estimating population access to insecticide-treated nets from administrative data: correction factor is needed. Malar J. 2013;12:259.
WHO. A framework for malaria elimination. Geneva: World Health Organization; 2017.
Hsiang MS, Ntuku H, Roberts KW, Dufour MK, Whittemore B, Tambo M, et al. Effectiveness of reactive focal mass drug administration and reactive focal vector control to reduce malaria transmission in the low malaria-endemic setting of Namibia: a cluster-randomised controlled, open-label, two-by-two factorial design trial. Lancet. 2020;395:1361–73.
Grossenbacher B, Holzschuh A, Hofmann NE, Omar KA, Stuck L, Fakih BS, et al. Molecular methods for tracking residual Plasmodium falciparum transmission in a close-to-elimination setting in Zanzibar. Malar J. 2020;19:50.
Bashford G, Richens J. Travel to the coast by highlanders and its implications for malaria control. P N G Med J. 1992;35:306–7.
WHO. Disease surveillance for malaria elimination. Geneva: World Health Organization; 2012.
Acknowledgements
The authors would like to thank all participatns in this study and colleagues at the National Department of Health, Provincial Health Authorities, and Rotarians Against Malaria (RAM) PNG for facilitating this study. We are also grateful to the PNGIMR field teams: T. Foropo, S. Gene, J. Moza, J. Tandrapah, M. Dreyam, I. Martin, W. Neiembe, E. Makoni, B. Kotuno, and the late Jessy Kara who is dearly missed. Special thanks to C. Goiye, F. Unga, for the administrative and logistical support of the project. This work was supported by Global Fund to Fight AIDS, Tuberculosis and Malaria (GF), and the Swiss Network for International Studies (SNIS). The funders has no role in study design, conduction of the surveys, analysis, interpretation, or the writing of the paper.
Funding
Open access funding provided by University of Basel. This work was supported by Global Fund to Fight AIDS, Tuberculosis and Malaria (GF), and the Swiss Network for International Studies (SNIS). The funders has no role in study design, conduction of the surveys, analysis, interpretation, or the writing of the paper.
Author information
Authors and Affiliations
Contributions
OS coordinated project management, data collection, analysis and developed the draft manuscript. SK, SJ, MK, EW,VS were responsible for fieldwork supervision and project coordination. OS and YU contributed to data management and analysis. LM, WP and MH were involved in the study design, interpretation and scientific guidance. MH reviewed the final manuscript. All authors read and approved the final manuscript. The opinions expressed in this paper are those of the authors and may not reflect the views of their employing institutions nor of their funders.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was reviewed by the Ethikkommission Nordwest- und Zentralschweiz (EKNZ), Switzerland (Req-2018–01063), and provided formal approval by the Institutional Review Board of PNGIMR (IMR IRB No.1808) and the Medical Research Advisory Committee of the National Department of Health (MRAC No. 18.22). Written informed consent was obtained from the head of the household and the school's head teacher. Further, individual verbal permission for finger-prick blood samples was secured from the participants or their guardians, as applicable.
Consent for publication
All authors consent to publication of this manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Seidahmed, O., Kurumop, S., Wawaga, E. et al. A combined school survey and reactive case detection reveals minimal local transmission of malaria in the Highlands Region of Papua New Guinea. Malar J 24, 3 (2025). https://doi.org/10.1186/s12936-024-05197-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-024-05197-2